Text Rianne Lindhout
Chicken farmers have been struggling with bird flu for years, and sheep farmers with bluetongue. But both may soon see an end to their problems. Wageningen Bioveterinary Research is testing vaccines for bird flu and bluetongue. Two researchers explain the process.
We have all read the distressing headlines or seen the pictures: ‘Bird flu: 130,000 chickens gassed’. You might be buying free range eggs but in the past couple of years these chickens too have often been kept indoors for months. Bird flu has regularly affected poultry and wild birds in the past, but since 2021 it has been circulating all year round.
This feared disease — that kills so many birds and is traumatic for farmers — may not be in the news much at present, but a lot is going on behind the scenes. A solution is in sight in the form of vaccines. They are currently being tested ‘in the field’ on two farms. Virus researcher Kim Bouwman of Wageningen Bioveterinary Research (WBVR) in Lelystad is closely involved. She lists some of her tasks: ‘I keep in contact with the animal keepers, the lab and the Animal Welfare Body. In consultation with our partners, I decide when to bring chickens from the farms to Lelystad to study the effectiveness of the vaccines.’ Bouwman started working for WBVR in October. Before she joined, two vaccines for bird flu — developed by pharmaceutical companies — had been successfully tested on 20 chickens in the lab. But that doesn’t give enough information on how effective the vaccines are. ‘The tests were done with chickens born and housed here. They weren’t exposed to the usual pathogens and other stress factors in the same way. That can influence the effectiveness of the vaccine.’
The researcher took charge of the field trials. They started last September with 1800 laying hens on two farms. ‘The hens were born in September. They were given all the standard vaccines plus one of the two bird flu vaccines we are testing. Laying hens usually live about 18 months. At four points during that period, we take a group of about 20 hens and infect them with bird flu in the lab. We will be doing the same with a control group of hens from the same farm that weren’t given the bird flu vaccine.’ It is already clear that the vaccine works well after eight weeks: none of the vaccinated chickens died, so the vaccines are a success in that sense. The second test has also been carried out. ‘We’re currently analysing the data.’
Acute problem
Even if the pilot project goes as hoped, the vaccine will still not be ready for authorization for commercial use. At least, not officially. Bouwman: ‘The pharmaceutical companies can submit an application to the Medicines Evaluation Board (CBG) based on the research results they already have; the CBG is the authority that decides on licensing for the European market.’ But if an acute problem arises in the form of a major outbreak, the CBG can issue emergency licences for vaccines. That is a similar situation to the COVID vaccines we humans had, where authorization went much faster than usual.
The urgency does make me even more enthusiastic about my work
If there isn’t an emergency situation in the form of a major outbreak, the two vaccines will have to be tested further after the current trial, on more farms. That study is already scheduled to start in August; WBVR is not involved. Bouwman estimates that this second pilot study will also take 18 months, the lifespan of the laying hens. The CBG would then be able to assess from February 2026 whether the vaccines should become available to everyone.
Bluetongue
Sheep farmers will be able to breathe a sigh of relief long before then. As early as this autumn they will probably no longer need to worry about bluetongue, a virus that killed tens of thousands of sheep last autumn after a plague of midges. Cattle don’t usually die from bluetongue, but they do get ill when bitten by a midge transmitting the disease, so there is a considerable economic impact on cattle farmers too.
In our animal stalls, they don’t get exposed to the usual pathogens and other stress factors
At WBVR, Melle Holwerda is in charge of the diagnostics for this disease and he advises policymakers. Over the past while, he has also been helping with the research on an effective vaccine. The results are now being considered by the same Medicines Evaluation Board (CBG) that will eventually decide on the authorization of the bird flu vaccines. But in other respects there are surprising differences compared with the development of the bird flu vaccines.
The bluetongue virus is more stable than the bird flu virus, which is constantly evolving slightly. That means the bird flu vaccine will probably have to be updated for each new outbreak, as is the case for human flu vaccines. There was previously no vaccine at all for bird flu, as it had not been permitted for the European market until spring 2023. The situation is different for bluetongue. There are 37 serotypes of this virus that barely change at all. Holwerda: ‘Last autumn, we had serotype 3. In 2006, it was the somewhat less virulent serotype 8 that was circulating. In winter 2007, a vaccine became available for that and we haven’t seen that virus since 2009.’ The midges will increase in number again in the autumn after a dip in the spring and summer. Serotype 3 is expected to reappear then, but Holwerda hopes all the sheep and cows will be vaccinated by that point.
I continue to take a scientific, impartial approach to my work
‘Last October, we set up an infection model in sheep, with funding from the Ministry of Agriculture.’ That means the researchers study exactly what happens in a group of sheep after an infection. They measure the fever, the numbers of virus particles in the blood and the immune response. The infection model shows the difference between sheep that the bluetongue virus makes ill and sheep that don’t have such symptoms. That is necessary for testing the effectiveness of the vaccines.
Then it was a question of waiting for pharmaceutical companies to commission WBVR to test vaccines. Holwerda: ‘At present, three pharma companies have vaccines that are authorized for use thanks to the cooperation of the Ministry of Agriculture. That doesn’t mean these vaccines have been licensed for the market in the European Union.’ That procedure takes longer and involves the above-mentioned CBG. In addition to WBVR, pharmaceutical companies can engage other players in Europe for the trials required to demonstrate the effectiveness of their vaccines. Which institute the pharma companies used is confidential information. At any rate, WBVR’s infection model was used for obtaining the authorization for use. Holwerda: ‘A vaccine only has a chance of being authorized if it has been tested on the target animal, which is sheep in the case of bluetongue.’
Level-headed
In contrast to the bird flu vaccine, farm sheep are used to test the bluetongue vaccines rather than lab-born animals. ‘A group of sheep are given the vaccine,’ explains Holwerda. ‘Then a few weeks later, after they’ve built up an immune response, they are infected with bluetongue serotype 3. We look at whether they react differently to the unvaccinated sheep in our model.’ Holwerda is not allowed to reveal any details about the results. ‘We send the raw data to the pharmaceutical companies and they then submit the application for authorization to the CBG.’
A vaccine only has a chance of being authorized if it has been tested on the target animal
How do the two vaccine researchers feel about having the fate of so many animals and farmers in their hands? They remain level-headed. Holwerda: ‘Of course I very much hope we’ll be able to prevent another outbreak in the autumn. But I continue to take a scientific, impartial approach to my work. I need to make sure the protocols are sound, but in the end it’s up to the CBG whether to accept the vaccines.’ Bouwman takes the same view. ‘The urgency does make me even more enthusiastic about my work. If we manage to get new vaccines, that will prevent so much suffering – for example, no more transport bans that stop farmers from selling their products, or in the worst case culls of barns.’
Very different vaccines for very different viruses
There are a lot of serotypes of the bluetongue virus, but they are relatively stable. The genetic material consists of ten double RNA strands, comparable to our own double-stranded DNA. Current bluetongue vaccines consist of complete but deactivated viruses. That means they don’t make the sheep or cow sick, but the animal does build up resistance to the active virus that is circulating.
Bird flu is a much less stable virus with single-stranded RNA. The RNA is divided into segments that the virus swaps with other virus particles during an infection. It’s a bit like sexual reproduction, really. The bird flu that WBVR is currently working on is called H5N1. H5 and N1 refer to two proteins. The vaccine for bird flu is essentially a piece of H protein attached to a harmless virus. Like all proteins in the bird flu virus, the H protein is liable to change, so the vaccines will probably need to be updated for each new outbreak. The H protein of the virus strain that is circulating, for example H7N2, will need to be incorporated in the vaccine.
H5N1 replicates in cattle respiratory tract cells
The highly pathogenic European bird flu H5N1 strains can replicate in lab-grown respiratory tract cells of cattle. That has been shown by research by WBVR, commissioned by the Ministry of Agriculture in response to recent H5N1 outbreaks among goats and cattle in the US. WBVR did infection tests with three different virus isolates from poultry and a fox. All three were able to replicate in the respiratory tract cells, although the virus titres dropped rapidly after three days. ‘That suggests the virus replication wasn’t very efficient,’ says researcher Luca Bordes, ‘but it doesn’t exclude the possibility of European H5N1 getting into dairy cattle.’ The bird flu virus has not yet been detected in European dairy cattle.

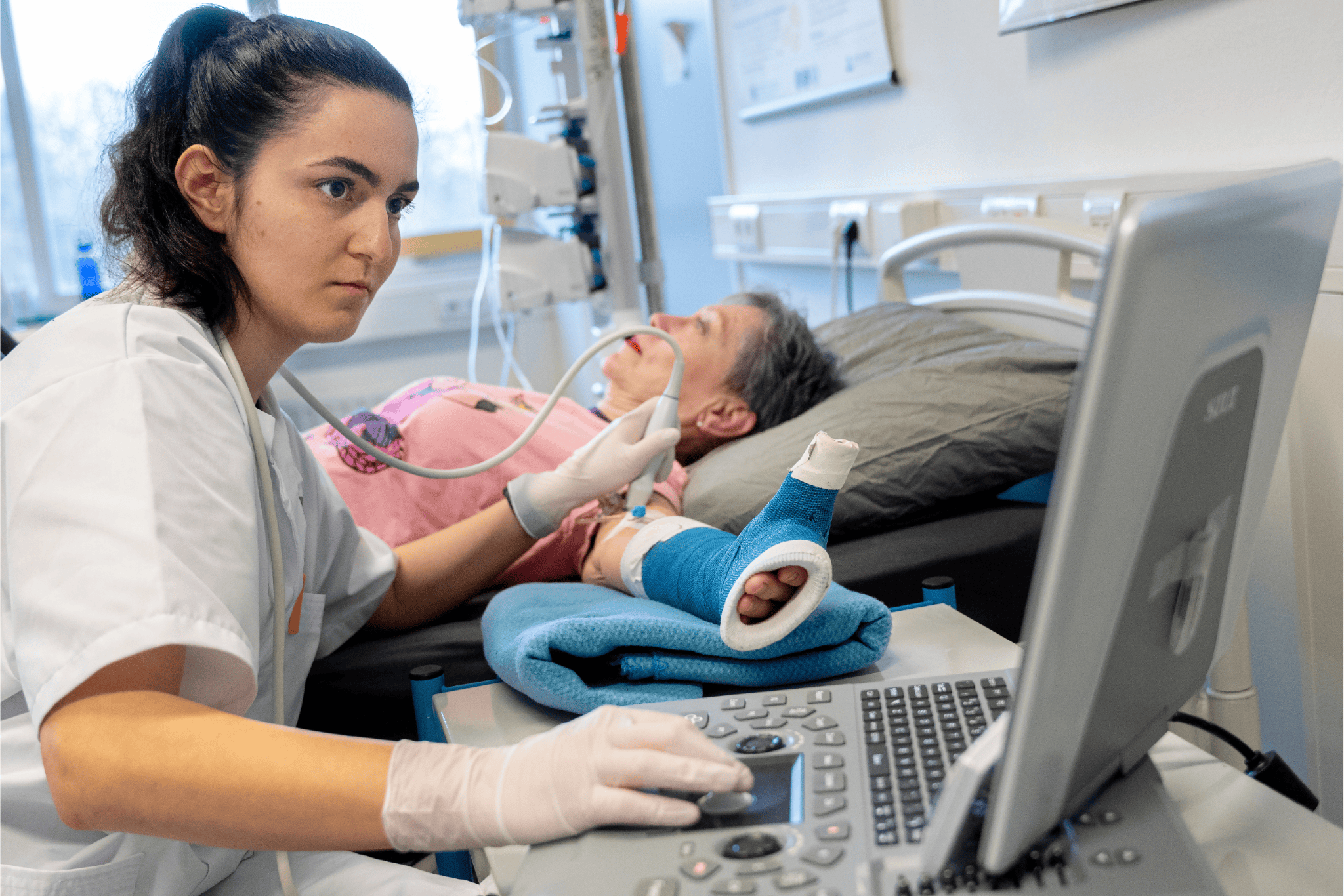
 Two vaccines for bird flu were shown in tests in WBVR’s lab to both protect the chickens against the disease and prevent it from spreading. Photo WBVR
Two vaccines for bird flu were shown in tests in WBVR’s lab to both protect the chickens against the disease and prevent it from spreading. Photo WBVR