The PFAS filter they developed has let organic chemists from WUR make great strides towards removing the harmful substance from the environment.
Was it serendipity, the chance discovery of something you are not even looking for, or was it by design, and the product of smart and calculated experimentation? Professor of organic chemistry Han Zuilhof stays neutral. ‘Either version would be too black-and-white. You have an idea and experiments produce results you didn’t expect. You continue to experiment on the basis of possible explanations. You all have different ideas, and together you construct the narrative.’ However it came about, the PFAS filter designed by Zuilhof and his team can rightly be called a triumph. The filter almost totally purifies water that is heavily contaminated with PFAS (see inset) in one simple step. To the extent that the water meets the quality standards for drinking water. What is more, the filter can be reused countless times. A single filtration suffices to reduce the concentration of PFAS in heavily polluted water to one millionth of what it was’
Cholera
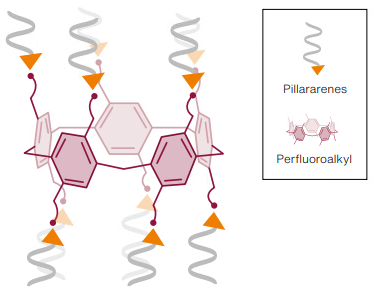
It all started with cholera, explains Zuilhof. ‘Cholera toxin, the protein complex that causes cholera, has fivefold symmetry and an additional toxic protein besides. The five identical locations let the toxin attach itself to cells. In 2016, we took the first steps towards making something like this ourselves with pillararenes, a class of large ring-shaped macromolecules. Pillararenes consist of five linked molecules with benzene rings and bridges that form a kind of pillar. We developed a chemical process for making those rings active by attaching molecules to them on both sides.’
At that point, PFAS molecules were not in the picture at all. ‘That changed when we started thinking about what we wanted to attach to those rings,’ explains Zuilhof. ‘It’s relatively easy to attach a positively charged molecule to them. One characteristic of this class of arene is that the “tails” with their positive charges at the end all point in the same direction, away from the pillar, along its axis.’ (See illustration.) That cloud of positive charges above and under the whole ring pull in negative charges like grippers. Take octanoic acid, the unfluorinated version of one common PFAS. Octanoic acid attached as expected. But things got really exciting, says Zuilhof, when the PhD researcher Tu-Nan Gao turned his attention to PFAS. PFAS proved to attach unexpectedly strongly, as if the grippers had been made for them. ‘The attachment is much stronger than with any other substance that can bind fluorinated compounds such as PFAS. At the same time, that attachment is much stronger than with any of the other substances in polluted water. Even if those other substances are present in concentrations a thousand times stronger. Those other substances attach somewhat as well, but then get squeezed out.’
Fluorine
Zuilhof now knows that the reason why the macromolecule binds PFAS so selectively is the presence of fluorine atoms in the carbon chains of the grippers. ‘Those fluorinated chains are lying side by side, which maximizes the interactions (known as Van der Waals forces, ed.) between the fluorinated chains. They form a kind of fluorine cloud that reinforces the binding of PFAS.’ Fluorine atoms maximize that binding force precisely because they are exactly the right size and positioned just right. And that paves the way for an important application as a PFAS filter.
This filter is formed by attaching one end of the pillar-shaped macromolecule to a fixed surface. The other end of the pillar grabs hold of PFAS in polluted water that is passed over the filter. Trials show that a single filtration suffices to reduce the concentration of PFAS in heavily polluted water to one millionth of what it was. That step takes less than five minutes. The filter can then be cleaned by rinsing it with alcohol, and it can be reused countless times.
You are still left with the question of what to do with the PFAS. Zuilhof’s team have come up with something for that too, and are working on a way of destroying the substances. But the research is not finished yet. PFAS is a collective term for thousands of compounds. Zuilhof’s PFAS filter fishes two important substances (PFOA and PFOS) out of the water. Zuilhof: ‘The question is whether we can adapt the filter so that other PFAS compounds are extracted too. We are working hard on that. There are also compounds that contain other halogens instead of fluorine. One example is bromine, which is used in fireproof materials. It might just work with bromine in the chain too.’
Zuilhof is understandably proud of the study, which was published recently. ‘My colleague Huub Rijnaarts once said there were two big problems with our water. We’ve either got too much or too little of it, and we’ve got PFAS. I can’t do anything about the volume of water, but I can do something about the PFAS. That triggered me. With our research, I can make a significant contribution towards addressing pollution with PFAS. That’s real science for impact. Great, isn’t it?’
PFAS
PFAS stands for per- and polyfluoroalkyl substances, a group of hydrocarbons containing a lot of fluorine. Well-known examples are PFOA (perfluorooctanoic acid, used in manufacturing Teflon) and PFOS (perfluorooctane sulfonates, used in the textile industry as a water- and stain-resistant preparation). PFAS are toxic for humans and the environment and do not biodegrade easily, if at all.
Patent
Professor Han Zuilhof’s group has taken out a patent on the method they developed for filtering PFAS out of solutions. Wetsus, the research institute for sustainable water technology, is going to market the patent. In exchange, one PhD candidate in Zuilhof’s group is going to do further research on cleaning up pollution with PFAS.

 For decades, the Chemours factory in Dordrecht emitted PFAS into the water and air. Photo Shutterstock
For decades, the Chemours factory in Dordrecht emitted PFAS into the water and air. Photo Shutterstock ![[The Proposition] ‘Environmental science enables a healthy environment in a nonstop polluting society’](https://www.resource-online.nl/app/uploads/2024/05/WEB_de-stelling.png)

