WUR researcher Louis de Smet and his group are working on advanced purification systems for extracting resources from wastewater. Progress is being made in this field.
De Smet is using an existing technique to desalinate water using electricity. If you dissolve salt (NaCl) in water and run it past two charged electrodes, the positively charged Na+ goes to the one and the negatively charged Cl– to the other. You can capture all the salts like this to obtain fresh water. But for De Smet, the water is only a by-product. His main aim is to fish specific molecules and salts, such as phosphate and lithium, out of the water in a pure form.
Electrodes
To desalinate water with this technique, electrodes in the form of carbon plates are usually used to store charged salt particles (ions). Once the carbon electrode is full of salt, you switch the poles around and it will release the salt again. This enables you to reuse the electrodes repeatedly.
Now De Smet wants to capture specific salts, and researchers are playing with the strength and duration of the electric field between the electrodes, as well as with the speed at which the water is pumped past the electrodes. They are also looking for membranes that allow the specific salts to pass through, and for alternative electrode materials with pores of a better shape and size.
Separation
The first steps have been taken towards combining two kinds of electrode material and a membrane in this kind of selection process, writes De Smet this month in the journal Chemical Engineering Journal. This will enable the researchers to separate double positive ions (such as calcium/Ca2+ and magnesium/Mg2+) from single ions such as sodium/Na+, while at the same time separating negative ions such as sulphate/SO42- from chloride/Cl–.
There has been no success yet, however, with isolating pure phosphate from wastewater. Phosphate is difficult because it is a large ion and if the electrode attracts it, smaller salts come with it too.
‘We don’t yet have a membrane that allows phosphate through specifically, although we are making progress in that area,’ says De Smet. This is likely to require a purification process involving several steps and techniques to make it sufficiently selective.
Lithium
The same applies to the process of fishing lithium out of wastewater. Lithium, used in batteries, is a small ion. You should be able to extract this ion from water with the right pore size in an electrode or membrane, says De Smet, but this too requires several techniques to work in tandem.
‘Researchers from several different disciplines are working on the technology for extracting valuable waste products from wastewater,’ says De Smet. He himself works at Organic Chemistry, where the main focus is on materials, and at the Wetsus water institute. At other universities, electrochemists, separation technologists and membrane specialists are working on the problem too.

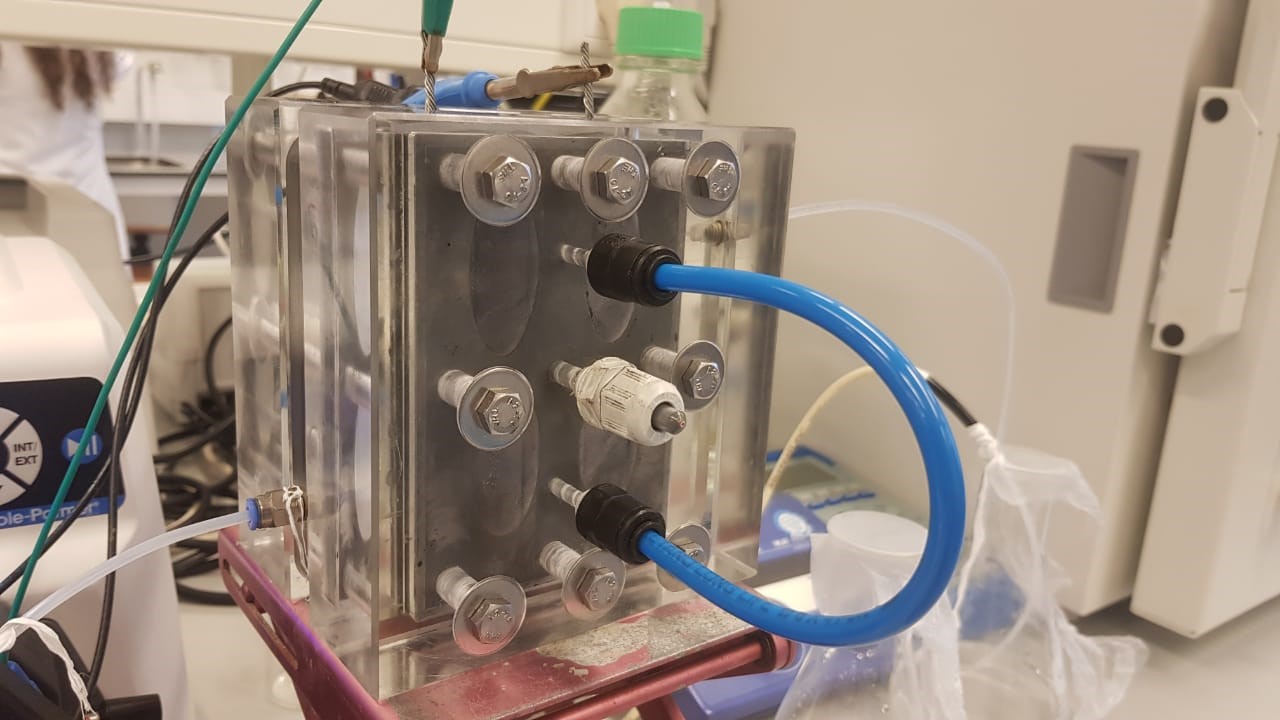 De Smet’s experimental apparatus, photo: Jayaruwan Gamaethiralalage.
De Smet’s experimental apparatus, photo: Jayaruwan Gamaethiralalage. 

