The Akkermansia bacterium was discovered in 2004 by Willem de Vos, Distinguished Professor at the Microbiology Group, during his search for commensal gut bacteria. Administering this bacterium to mice helps in combating overweight, and supposedly also other diseases, such as fatty liver disease and bowel diseases. In 2016, De Vos and Belgian professor Patrice Cani of the UCLouvain established the company A‑mansia Biotech, which aims to market the bacterium as a food supplement for humans.
Test groups
They have now completed a human pilot trial together with researchers of the Louvain Drug Research Institute. ‘The tests confirm what we had already observed in mice’, Willem de Vos tells enthusiastically. They gathered 32 volunteers who have a higher risk of developing cardiovascular diseases and diabetes (type 2), due to varying causes, including overweight, obesity or insulin resistance. The volunteers were randomly split into three groups and were administered either a placebo, living Akkermansia bacteria or pasteurised (killed) bacteria in the form of food supplements over a period of three months.
This does not mean that the bacterium cures cardiovascular diseases, but it does mean that the risk to develop these could be decreased.
Willem de Vos, Distinguished Professor of Microbiology
Differences with control group
‘The main goal of the study was to demonstrate that it is safe and feasible to administer the bacterium to humans on a daily basis in the form of a food supplement’, De Vos explains. This turned out to proceed very well. Moreover, the scientists discovered that certain risk factors for developing cardiovascular diseases, such as overweight, insulin resistance and a high level of cholesterol, had increased less and in some cases even decreased in people who had been administered the bacterium for three months – both alive and pasteurised, while the control group’s status only deteriorated. De Vos: ‘This does not mean that the bacterium cures cardiovascular diseases, but it does mean that the risk to develop these could be decreased.’
Follow-up
The researchers would like to follow up with a large-scale and long-term human trial, to see whether it could confirm the results and broaden their understanding. De Vos hopes to market a food supplement within two years. ‘It is not a quick fix’, he warns. ‘By administering the bacteria, we can move the tipping point a bit, which could defer the development of overweight. But sufficient activity and healthy meals remain very important.’

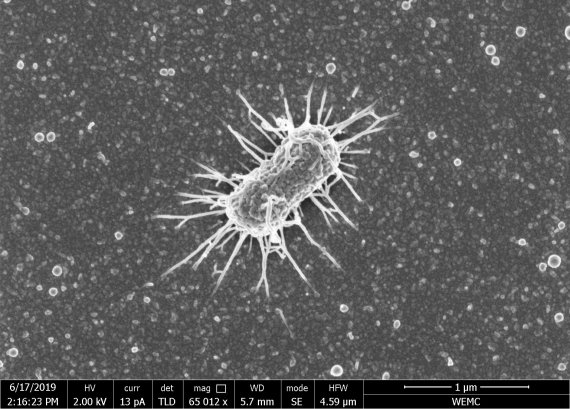 Akkermansia muciniphila seen through an electron microscope. Photo: Willem de Vos
Akkermansia muciniphila seen through an electron microscope. Photo: Willem de Vos